HIV Transmission in the Female Genital Tract
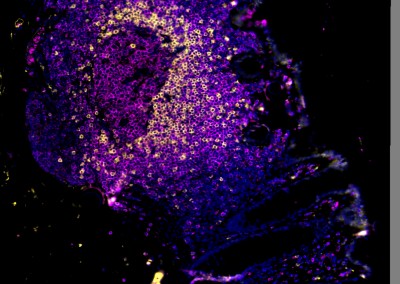
Young women bear a disproportionate burden of the HIV epidemic, with HIV prevalence rates up to eightfold higher in young women compared to young men in many sub-Saharan African countries. In the majority of new infections, the mucosal tissue of the female genital tract (FGT) is the primary site for HIV infection and the initial site of viral replication. A better understanding of the impact of the immunological environment of the FGT on HIV risk is therefore crucial to the development of new strategies to halt HIV transmission in women, particularly those living in sub-Saharan Africa.
Results from a number of studies suggest that elevated FGT inflammation increases risk of HIV acquisition. Microbially-driven sexually transmitted infections (STIs), bacterial vaginosis (BV), and reproductive hormones are known modulators of genital inflammation and increased HIV acquisition risk. We have applied next generation sequencing technologies to characterize the vaginal microbiome in young women in sub-Saharan Africa and demonstrated that the majority of these women have microbial communities that are very different from those of women living in resource rich settings. We further demonstrated that specific communities drive genital inflammation and result in an over 4-fold increased risk of HIV acquisition. This suggests that the vaginal microbiome may be leveraged to reduce HIV acquisition in young women in sub-Saharan Africa.
The characterization of the vaginal microbiome to date has largely focused on the bacterial component, however other domains of life, including fungi, viruses, and potentially as-yet-incompletely-defined organisms, inhabit the FGT and may contribute to inflammation. We have therefore moved beyond characterization of bacteria by using shotgun sequencing to comprehensively characterize other microbial populations within the FGT. Furthermore, the majority of microbiome studies, including those focusing on the FGT, have been performed on women of North American or European descent. This raises questions of how informative previous vaginal microbiome studies are to the identification of the microbial drivers of inflammation in the FGT of women living in different geographies. Several gut microbiome studies have shown strong geographical trends in bacterial sub-species structures whereas others have shown a host genetic component to microbial community structure. We are therefore studying women living in a number of different geographies and performing strain profiling of the vaginal microbiome. We are combining this with studies of microbial function, bacterial engineering, and deep characterization of host microbial sensing and mucosal immunity to develop interventions to reduce HIV infection in women.

HIV Disease Progression in the Gut
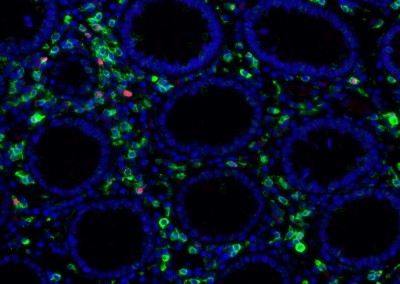
The human gut harbors an enormously diverse community of microorganisms collectively referred to as the “microbiome”. This microbiome has co-evolved with humans over half a billion years to assist in critical host metabolic and immune function. The impact of changes in the microbiome on disease states as diverse as diabetes, obesity, inflammatory bowel disease, epilepsy, and multiple sclerosis is only now being recognized. It is clear that human immunodeficiency virus (HIV) infection has profound effects on the intestinal mucosal environment with one of the hallmarks of HIV infection being a rapid and profound depletion of CD4+ T cells within gut associated lymphoid tissue (GALT). Despite this, there is a paucity of information regarding the changes that occur in the intestinal microbiota in HIV infection and the potential effects of these changes on host immunity and HIV disease progression. It is believed that HIV infection results in intestinal inflammation and increased intestinal mucosal permeability with subsequent translocation of luminal microbial products into the systemic circulation. This microbial translocation drives chronic immune activation, which results in T cell exhaustion and HIV disease progression. The mechanism by which HIV induces intestinal epithelial dysfunction and microbial translocation remains incompletely understood. Even less is known about how gut microbial populations may influence, or be influenced by, this process. Still, it has been shown that enteric microbiota in humans and nonhuman primates can be significantly altered early in infection by lentiviral-mediated disease. Abnormal microbiota are strongly associated with intestinal inflammation in inflammatory bowel disorders with data suggesting a causal role in gut inflammation. This suggests that the microbiome may play a direct role in mediating intestinal dysfunction. It is therefore critical to develop a more detailed understanding of the impact of alterations in the intestinal microbiome on HIV-associated intestinal dysfunction and disease progression.
Our team aims to understand how HIV-related defects in vitamin A metabolism and intestinal epithelial homeostasis may contribute to immune dysfunction and disease progression. Using peripheral and tissue samples from well-characterized cohorts of HIV-infected individuals, we analyze biomarkers and cell populations by flow cytometry, microscopy, advanced single cell sequencing approaches and in vitro and in vivo models to mechanistically dissect ex vivo observations from our human cohort studies. We are performing this characterization of host mucosal immune function with coordinated studies of the gut microbiome, to better understand how HIV impacts both enteric microbial communities and host immune function to better understand the role of the gut in HIV disease progression.
HIV/TB Co-infection in the Lung
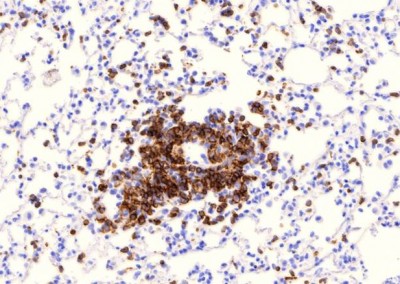
It is estimated that one third of the world population is infected with Mycobacterium tuberculosis (M. tb) and although active tuberculosis (TB) was in decline at the end of the 20th century, there was a sharp increase coincident with the emerging HIV epidemic in sub-Saharan Africa. Between 1990 and 2008 the number of TB cases in this region increased by 40%, mostly due to the increasing prevalence of AIDS. The risk of active TB is 5-10% in a lifetime for HIV uninfected individuals and 10%annually for those who are HIV-positive. Antiretroviral treatment (ART) leads to control of viral replication and recovery of CD4 T cell numbers but does not eliminate the increased risk of active TB. TB is now the leading cause of death among those with HIV. Activated macrophages are thought to be critical to the control of intracellular mycobacteria and although the mechanisms of macrophage anti-mycobacterial effector function are not completely understood it has been proposed that Vitamin D plays a pivotal role in initiating mycobacterial killing. In vitro studies with human monocyte derived macrophages (MDMs) demonstrate that stimulation with CD40L, IFN-γ or TLR ligands such Pam3CSK increases expression of the Vitamin D receptor (VDR) and the enzyme Cyp27b1, which converts Vitamin D to its active form. Presence of active Vitamin D induces expression of potent mycobacterial pathways including anti-mycobacterial peptides such as LL37 and HBD2 and the anti-bacterial autophagy pathway. Therefore the Vitamin D pathway appears to be crucial for human MDM control of mycobacterial growth in vitro. HIV infection of MDMsin vitro appears to impair the ability to activate the Vitamin D pathway. However, the importance of these pathways in primary alveolar macrophages and lung resident tissue macrophages from healthy and HIV infected individuals is yet to be established.
We are performing studies assessing the effects of HIV on lung macrophages collected by bronchoscopy from patient cohorts in the U.S. and sub-Saharan Africa. We are defining the specific defects in lung macrophage function that are induced by HIV infection to better understand why individuals with HIV have impaired immunity against M. tb. We are combining this work in patients with studies using in vitro and in vivo models of HIV and M tb co-infection, to identify novel mechanisms by which HIV contributes to increased M tb susceptibility.
Rapid Diagnostics
In the U.S., over 2.2 million people each year have a serious infection caused by antibiotic resistant bacteria. This results in over $20 billion in excess healthcare costs and more than 8 million additional days spent by patients in the hospital. A major contributing factor is the outdated way in which we currently diagnose infections. Current methods rely upon culture, but this process is inherently slow and inefficient, requiring 3-5 days from the time of sampling to a final identification of the causative bacterial species and the antibiotic susceptibility of the isolated pathogen. Even more worrisome, in about 15% of cases cultures fail to grow because of prior treatment with antibiotics or inability to efficiently culture the causative pathogen. In the intervening time, doctors are forced to administer empiric therapy with broad-spectrum antibiotics, which result in side-effects for the patient and an increased rate at which bacteria evolve drug resistance.
We believe that a culture-free molecular approach using next-generation sequencing will allow drug resistant infections to be identified within hours rather than days. We are developing a process to perform pathogen enrichment from clinical material and combining this with the development of computational strategies that take advantage of recent developments in machine learning to predict drug resistance directly from bacterial DNA reads, without the need for assembly or a reference genome. Our approach will result in the ability to identify specific bacterial species and antibiotic susceptibility from a clinical sample in a matter of hours, rather than days. This will be transformative for the practice of medicine, and will result in the reduction of antibiotic resistance and a significant improvement in our ability to care for patients. Find out more information at our spin-out biotech, Day Zero Diagnostics.